The National Institute of Health estimates that approximately 65% to 80% of microbial infectious diseases are associated with bacterial biofilm [1]. Biofilms are defined as structured communities of bacterial cells enclosed in a self-produced polymer matrix that is attached to a surface, allowing for greater protection of bacteria to thrive in potentially hostile environments [2]. Biofilm-associated bacteria complicate both human and veterinary medicine, as these pathogens can cause chronic infections and are highly resistant to antibiotic therapy [2].
With antibiotic-resistant strains of bacteria on the rise, veterinary researchers have begun to take a closer look into the mechanisms that cause biofilm to proliferate in chronic bacterial infections. If you suspect your pet is suffering from chronic infections, it is recommended that you make an appointment with your veterinarian to diagnose and provide a treatment plan.
How does Biofilm Develop & What Does That Mean for My Pet’s Treatment?
Genetic studies have revealed the complex process by which biofilm grows, as the development of these microbial communities requires a unique type of communication between bacteria known as quorum sensing [3]. Interestingly, biofilm development by a species of bacteria requires transcription of a set of genes that is unique from transcription by the singular bacterium, indicating that bacteria alter their physiology dependent on the presence of other bacteria [3].
In short, the formation of biofilm is complex, with its development characterized by five distinct phases: 1) development of a surface conditioning film; 2) reversible and irreversible attachment of cells to a surface; 3) formation of microcolonies; 4) maturation and differentiation of the biofilm; and 5) detachment and dispersion of cells from the biofilm [1].
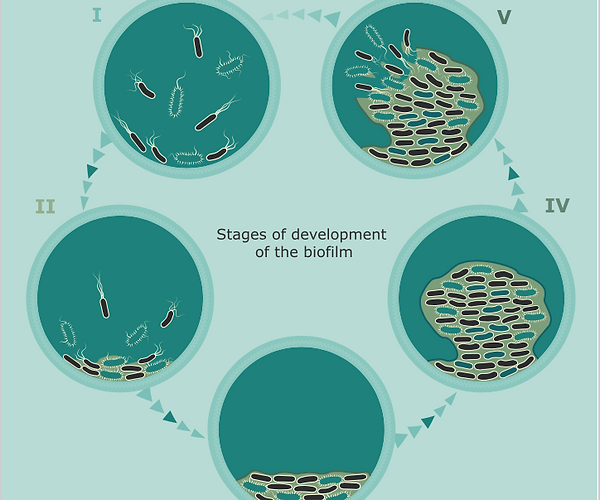
The five stages of biofilm development.
Antibiotic Resistance and Biofilms
Compared to freely floating, planktonic bacteria, biofilm is much more resistant to antimicrobial agents and in most cases requires over one thousand times more antibiotics for treatment as opposed to its free-floating counterparts [1]. Biofilms can provide bacteria protection against altered pH, osmolarity, lack of nutrients, and physical disturbances [4]. Biofilm is able to tolerate various antimicrobial compounds due to its intrinsic genetic and physiologic diversity. Some bacteria in biofilm may also protect other microbial members from antimicrobial compounds through the expression of efflux pumps that expel antibiotics from cells, allowing the community to grow even in the presence of antibiotics [4]. Notably, biofilms create a physical barrier between antibiotics and the biofilm community. This matrix consequently bolsters the resistance of bacteria and allows them to withstand both harsh conditions and antimicrobial compounds, resulting in the rise of infections that are either greatly or fully resistant to one or more antibiotic treatments.
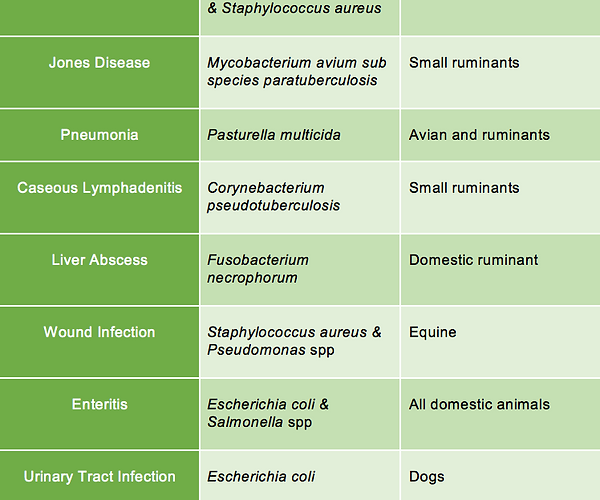
In a systematic review, researchers compiled a condensed list of common microbial diseases in pets that are complicated by biofilms [1]. It is important to note that this list is not comprehensive and simply illustrates common biofilm-associated infections. The list can be seen in the table below:
Table 1. Common biofilm diseases in veterinary medicine adapted from Abdullahi et al. 2016.
Treating and Diagnosing Biofilm-Associated Infections
Treatment for biofilm-associated infectious diseases requires aggressive therapeutic approaches. However, before treatment, it is critical to understand the extent along with antibiotic resistance present of a biofilm infection. Historically, culture-based methods have been used to assess the microorganisms within a biofilm, but this diagnostic approach provides an extremely limited picture of the true nature of a biofilm [5]. Biofilms commonly remain completely undetected by modern culture methods, as only <1% of microorganisms can be cultured. Moreover, biofilms cannot be cultured at all. Various studies have confirmed this dilemma, as a biofilm culture “often results in [a] negative culture result, [and] in some cases false positive result is obtained due to the presence of free moving planktonic bacteria” [1].
One of the major problems in using culture-based methods to characterize biofilm is that the media in which cultures are performed is completely different than the natural systems in which biofilms develop. Planktonic bacteria that are cultured have access to more nutrients and are able to proliferate at much faster rates, but are more responsive to antimicrobial compounds [6]. Conversely, bacteria growing in natural biofilm settings may have less access to nutrients and thus grow more slowly, but are less susceptible to antimicrobial compounds and environmental factors [6]. The ability of biofilm to quorum sense, to have a physical barrier between antimicrobial compounds, and more, creates a completely different context for biofilm-associated bacteria. When bacteria are examined in natural settings and tissue of interest, biofilms are the most predominant form, and so to base diagnostics off of culture-based methods would not provide a full picture.
Treatment of biofilm infections requires a combination of antibiofilm and antimicrobial agents [1]. Consult with your veterinarian if you have concerns your pet is suffering from a chronic infection, as a biofilm-targeted therapy may be useful. Modern diagnostics, particularly in Next-Gen Sequencing (NGS) have allowed veterinarians to create more targeted treatments for chronic, biofilm-associated infections.
NGS has increasingly helped researchers and veterinarians characterize biofilm. A recent study assessing the composition of biofilms in chronic wound infections of dogs also affirmed the applicability of NGS technology in characterizing biofilm-associated pathogens [7]. This indicates the clinical applicability of using genomic sequencing to identify, analyze, and eventually treat animals more effectively.
The MiDOG All-in-One Microbial Test offers comprehensive diagnostic information for pets suffering various biofilm-associated infections. Utilizing NGS technology to detect and quantify all microbial DNA through untargeted and comprehensive sequencing and quantitative comparisons to reference databases, the MiDOG NGS technology provides a useful opportunity to shed light on the microbial makeup of your pet’s infection for clinical application. The MiDOG microbiome test is a microbial identification test grounded on scientific research that provides veterinarians DNA evidence for the guided treatment of biofilm-associated infectious diseases.

Find out if your vet uses MiDOG before you book your next appointment!
For health-related questions about your pet, reach out to a veterinarian.
References
[1] Abdullahi, U., Igwenagu, E., Mu’azu, A., Aliyu, S., & Umar, M. (2016). Intrigues of biofilm: A perspective in veterinary medicine. Veterinary World, 9(1), 12-18. doi: 10.14202/vetworld.2016.12-18
[2] Marcinkiewicz, J., Strus, M., & Pasich, E. (2013). Antibiotic resistance: a “dark side” of biofilm‑associated chronic infections. Polish Archives Of Internal Medicine, 123(6), 309-313. doi: 10.20452/pamw.1780
[3] Jamal, M., Ahmad, W., Andleeb, S., Imran, M., Nawaz, M., Hussain, T., Ali, M., Rafiq, M., Kamil, M. (2018) Bacterial biofilm and associated infections. Journal of the Chinese Medical Association, 81(1), 7-11. https://doi.org/10.1016/j.jcma.2017.07.012
[4] Sharma, D., Misba, L. & Khan, A.U. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8, 76 (2019). https://doi.org/10.1186/s13756-019-0533-3
[5] König, L., Klopfleisch, R., Höper, D., & Gruber, A. (2014). Next Generation Sequencing Analysis of Biofilms from Three Dogs with Postoperative Surgical Site Infection. International Scholarly Research Notices, 2014, 1-5. doi: 10.1155/2014/282971
[6] Olson, M. E., Ceri, H., Morck, D. W., Buret, A. G., & Read, R. R. (2002). Biofilm bacteria: formation and comparative susceptibility to antibiotics. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire, 66(2), 86–92.
[7] Wu, H., Moser, C., Wang, HZ. et al. Strategies for combating bacterial biofilm infections. Int J Oral Sci 7, 1–7 (2015). https://doi.org/10.1038/ijos.2014.65
Categories: Antibiotic Resistance, Dogs, Next-Gen DNA Sequencing Technology, Pet Health, Safety and Wellness, Pet Parents

